Questions & Answers
Neuren Pharmaceuticals is a specialty biopharmaceutical company with a focus on rare, neurodevelopmental disorders. Our lead medicine, Trofinetide showed strongly positive results in a Phase III study in Rett syndrome, meetings its co-primary endpoints and demonstrating statistically significant improvements over placebo. We now have NNZ-2591 in Phase II trials across 4 disorders, including Angelman syndrome.
Learn more about us at our website: www.neurenpharma.com.
Clinical Trials provide information to determine whether a medicine is safe and effective. Regulatory Agencies worldwide require the data from such clinical trials to assess a medicine’s benefit to the population being treated.
Clinical trials are carried out in humans after laboratory and animal testing demonstrates that a treatment is likely to be efficacious and safe. The trials move through a series of phases (I, II , III and IV), and follow protocols (approved by Ethics Committees) that balance potential risks with benefits.
All participants in this study will receive thorough study-related physical, psychological, and behavioural evaluations by a study doctor.
While there is no guaranteed benefit to participants in a clinical research study, participation may help to advance scientific knowledge in the area that is being studied.
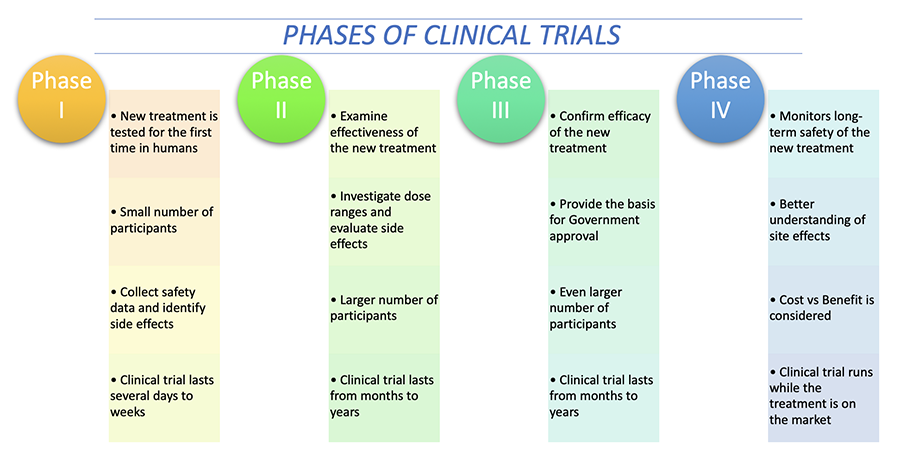
The phases of clinical trials are the stages where researchers conduct experiments to find out if a certain medicine or intervention is considered efficacious as a new medical treatment. These range from Phase 1 to Phase 4. The image below summarises the four phases.
In Phase 2 studies like this, researchers administer the study medication to a group of patients who have the condition for which the medicine is being developed.
The next stage is Phase 3, where researchers will confirm the efficacy of the new treatment.
A protocol outlines the plan for the clinical trial. It is carefully designed to protect the wellbeing of the participants and answer specific research questions. A protocol outlines who can participate in the trial, when different assessments should be taken, which procedures will be done, details of medications and how much to give, as well as information on the duration of the study. While participating in a clinical trial, participants are seen regularly by the study team to monitor their condition and determine the safety and efficacy of the treatment.
Once a site has received Ethics Committee and Research Governance approval for the study, you will be able to contact them directly to discuss your child’s potential eligibility. They will arrange a series of screening visits that will assess and confirm that your child meets all the eligibility criteria detailed in the protocol. These criteria are based on age, type of condition, medical history, concurrent medications and a range of clinical and behavioural assessments. If your child meets all these criteria, they will be eligible to participate in the study.
The current study for children and adolescents with Angelman syndrome is a Phase 2 trial. This trial will include up to 20 male and female participants between the ages of 3-17 years. Your participation will last for approximately 19 weeks.
The study primarily examines the safety profile of the study medication in children with Angelman syndrome. NNZ-2591 has already been tested in healthy human volunteers for 7 days (the Phase 1 study) and was shown to be generally safe and well tolerated in that study.
This Phase 2 study is intended to confirm that NNZ-2591 is a well-tolerated medicine that is worthy of further development for children and adolescents with Angelman syndrome. This study is also measuring efficacy (whether individuals see improvement or benefit from taking the study medication). It includes a number of assessments that look at a number of areas of concern in Angelman syndrome such as overall well-being, communication, behavior, and quality of life.
To be eligible for the study, the participant must have a clinical diagnosis of Angelman syndrome together with documented evidence of genetic background known to impact maternally derived UBE3A expression in the brain. This includes deletions or mutations of the UBE3A gene, UPD or imprinting. However, children with mosaic presentations are not eligible.
Our main goal in this study is to examine the effectiveness of the new study medication (NNZ-2591) in the treatment of Angelman syndrome. This means that if your child has been diagnosed with multiple genetic disorders, unfortunately they are unable to participate in this study. Many of these disorders have overlapping symptoms, and it would be impossible to accurately measure the effect of the study medication on the intended indication, in this case Angelman syndrome. If you have specific questions or concerns, please speak with your study doctor.
Having the COVID-19 vaccine does not affect your child’s eligibility to participate in this study. Nor is it an issue if received part-way through the study. We’d advise having the vaccination ahead of participation in the trial to avoid any potential confusion over side effects. The study team will record any vaccines or medications that have been received during the treatment period.
At this stage, our development program is focused on paediatric patients, however, we are planning an adult development program. Timings for this adult program have not yet been defined.
Blood samples will be taken by skilled study nurses who have extensive experience working with children who have neurodevelopmental disorders, specifically Angelman syndrome.
The ethical and legal codes that govern medical practice also apply to clinical research. All clinical research has built in safe guards, including independent ethics committee review and approval with built in safeguards to protect the participants’ information. The trial follows a carefully controlled protocol, a study plan which details what researchers will do throughout the study. Safety data is reviewed as a clinical trial progresses. Researchers report the results of the trial at scientific meetings, to medical journals, and to various government agencies. Individual participants' information will be de-identified to remain anonymous and will not be mentioned in any reports.
Yes, a participant can choose to withdraw from a clinical trial at any time. You should speak to the study team to let them know you no longer wish for the participant to continue in the trial.
Unfortunately, we’re unable to provide access to the study medication once your participation has ended. The medicine has not yet been studied (in either humans or animals) beyond 13 weeks of dosing, and so we would not have Ethics Committee approval to proceed beyond this timeframe.
You can participate in the study; however, this participation will obviously be subject to the relevant Covid-19 border restrictions between your state and whichever site you choose.
Flexibility has been built into the study protocol and supporting structures to allow for continued subject participation during the Covid-19 pandemic. However, to ensure the integrity of the study, there are a number of study visits where it is critical that on site attendance occur. If you live interstate, you will need to consider the effect that state-based lockdowns may have on your ability to participate and travel.
Yes, once confirmed as eligible, support for attendance at in-clinic visits is available. Where required, you can be reimbursed for your reasonable travel and accommodation costs. The process will be managed by the Angelman Syndrome Association Australia (ASAA) and please visit their site for details on how to participate.